The new Alzheimer’s drug causes excitement and concern
By now, you have probably heard about the new Alzheimer’s drug that was recently approved by the FDA. It was developed by the drug company Biogen and is called aducanumab (brand name Aduhelm). This is the first new Alzheimer’s drug in18 years, which is a big deal, considering that in the United States, 6.2 million people are living with the disease. Even more significant is that it targets underlying disease, not just symptoms.
The problem is, not everyone thinks approving the drug was the right thing to do. The FDA’s Advisory Board recommended against it saying that trials had produced little evidence it was effective. When it was approved anyway, three members of the board resigned in protest.
The drug has been controversial from the start. Biogen stopped Phase 3 studies because they failed to meet primary outcomes. But in October 2019, it reversed its position, saying that further analysis of the data from one of those studies showed that high doses of the drug may actually slow cognitive decline in people with early Alzheimer’s or mild cognitive impairment. The company decided to move ahead and apply for accelerated FDA approval.
According to the FDA, researchers evaluated Aduhelm’s effectiveness in three separate Biogen studies that represented 3,482 patients with Alzheimer’s disease. Patients who got the drug “had significant dose-and time-dependent reduction of amyloid beta plaque, while patients in the control arm of the studies had no reduction of amyloid beta plaque.” Based on those findings, the FDA granted its approval, with the condition that Biogen must conduct Phase 4 follow-up studies to monitor for serious side effects, including brain swelling and bleeding.
Drew Wyman, Executive Director of the Maine chapter of the Alzheimer’s Association, says they were thrilled with the decision.
History has shown that approval of a first drug in a new category stimulates the field and invigorates researchers. It results in innovative thinking, thinking outside the box, developing new ideas, and building on other research. The Alzheimer’s Association’s position is that we should be looking at everything. Leave no stone unturned. You don’t want to just discredit any theory, but you also don’t want to chase one theory. It simply opens up the floodgates and that is the impetus for our excitement.
Drew Wyman, Executive Director, Alzheimer’s Association, Maine Chapter.
Dr. Eric Dinnerstein, a cognitive neurologist at MaineHealth, agrees that the FDA approval is a historic event.
I can’t over-describe how historic it was. This is the first medicine in history to target Alzheimer’s as a potential disease-modifying agent. No other medicine was targeting the actual cause of the disease.
Eric Dinnerstein, MD, Neurologist, MaineHealth
As exciting as it may be, Dr. Dinnerstein also has some concerns about the FDA’s decision to approve the drug.
I’m worried that patients will want it and I won’t be able to prescribe it if it is limited to small groups of patients. I’m worried about the very hard decision-making of who to give it to. I’m worried that I’ll encounter one obstacle after another. The health insurance companies are going to have to approve this, which might be a big barrier. Would you pay $50,000 a year to get a medicine that will slow down your progression by 20%? It’s not only the cost of the medicine, it’s three or four visits and MRIs a year, which adds up. Then there’s the issue of patients dropping out of clinical trials, wondering why they should participate in a placebo-controlled trial with another medicine. They will ask me, do you know that the other drug is better? I would have to say I don’t. I am worried, but also excited. I’m allowed to be both.
Dr. Dinnerstein
In full disclosure, Dr. Dinnerstein is currently the principal investigator for a clinical trial on a drug similar to aducanumab called donanemab. The trial is run by the competing drug company Eli Lilly. He is also on the board of the Maine Chapter of the Alzheimer’s Association. The Association and other patient advocacy groups lobbied hard for the drug to be approved, another thing that worries him.
The whole concept of the FDA sitting on difficult decisions and public groups, many of them non-scientific based, putting pressure on it, is a matter of concern.
Dr. Dinnerstein
Will he give the drug to his patients?
Yes. I want to help my patients, and if there’s something that might help, I want to give it to them. Who am I to be the decision-maker that a 20% decline in the progression of a disease is good or not good?
Dr. Dinnerstein
So … how does aducanumab work and how is it different from the other Alzheimer’s treatments on the market? Let’s begin by taking a look at what we know about what happens in the brain of someone with Alzheimer’s disease.
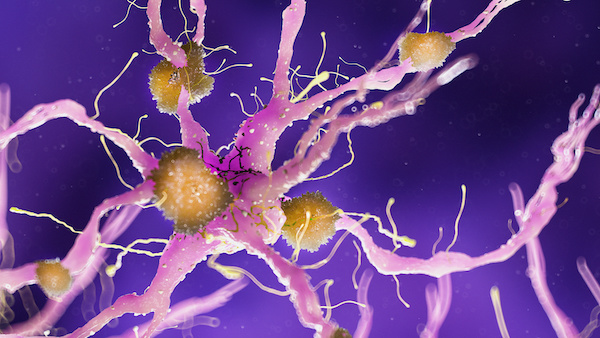
The biology of Alzheimer’s disease
Researchers believe that Alzheimer’s develops from multiple factors, including genetics, lifestyle, and environment. The disease causes brain cells to die and brain tissue to shrink. The damage seems to come from two sources: One is a protein called amyloid. Abnormal clusters of protein fibers, called amyloid plaques, build up between the brain’s nerve cells. They may also activate the immune system and trigger inflammation.
The second source is another protein called tau, which helps transport nutrients and other important substances from one part of a nerve cell to another. In the brains of people with Alzheimer’s disease, tau proteins are abnormally shaped or folded wrong. They look tangled. Together, these two damaged proteins can cause a lot of damage, although amyloid plaques can also be found in the brains of people without Alzheimer’s.
Treating symptoms
Scientists have been focused on finding a way to target the amyloid for the past 20 or so years. The drugs that have been used don’t; they only treat Alzheimer’s symptoms.
Certain medicines seem to stimulate or boost brain activity. Medicines like Donepezil (Aricept), Memantine (Namenda), Galantamine (Razadine), or Rivastigmine don’t work on the plaque, they work on neurotransmitters that connect cells to each other. It’s like a high-speed Internet if you will. It helps the brain cells communicate and interact in a more efficient way.
Dr. Dinnerstein
All the while a patient is on one of these medications, the amyloid plaques are accumulating, and the disease continues to progress. Eventually, the drugs no longer work.
Treating the disease
The goal with aducanumab and similar experimental drugs is to remove the amyloid or prevent it from accumulating. Aducanumab is a monoclonal antibody that attacks the plaque by binding to it and sending a signal to the immune system to destroy the plaque.
In the past 20 years, we found very efficient ways of removing the amyloid plaque from the brain. We’ve proved it with certain brain scans and so forth. It’s quite exciting, but we’ve also been disappointed because, in waves of trials, patients continued to deteriorate. However, patients with milder symptoms did better, and that is what led us to today.
Dr. Dinnerstein
While the drug seemed to benefit people with mild cognitive impairment, the FDA approved it for patients at all stages of Alzheimer’s disease. However, if it is a cascade of events that causes the disease, the earlier you can interrupt that cascade, the better. At least, theoretically.
I like to use the metaphor of lighting a match. The amyloid would be the match. Once the process has started and the fire is lit, even if you remove the match, it might be too late.
Dr. Dinnerstein
Early signs of Alzheimer’s
If earlier really is better, what risk factors or symptoms of cognitive decline should you be on the lookout for? You’ll find a wealth of information on the Alzheimer’s Association’s website, but you can start with these 10 early signs.
- Memory loss that disrupts daily life.
- Challenges in planning or solving problems.
- Difficulty completing familiar tasks.
- Confusion with time or place.
- Trouble understanding visual images and spatial relationships.
- New problems with words in speaking or writing.
- Misplacing things and losing the ability to retrace steps.
- Decreased or poor judgment.
- Withdrawal from work or social activities.
- Changes in mood and personality.
An accurate, early diagnosis is really important so that we can measure the data correctly and administer things correctly. We need to educate physicians, professionals, family members, community members, everyone about the warning signs and symptoms and what to do. That’s why the association is so robust in its awareness and educational programming.
Drew Wyman
Screening and assessment tools are extremely valuable, but the only way to diagnose Alzheimer’s is by sampling spinal fluid with a spinal tap or having an Amyloid PET Scan. As an aside, the Alzheimer’s Association funded the study that resulted in the development of this PET scan.
That’s one way the association ties it together. This drug could not have been developed without the Alzheimer’s Association funding the Amyloid PET Scan breakthrough. That’s part of why we’re excited. It piggybacks on other research initiatives.
Drew Wyman
More information
If you’re concerned that you or a loved one has any of the warning signs or you want more information about aducanumab (Aduhelm), reach out to your healthcare provider. You can also contact the Alzheimer’s Association 24/7 HELPLINE at 800.272.3900.

Is this the right quote for Eric Wyman above?
“An accurate, early, inaccurate diagnosis…”
Jeffrey, it was my mistake and I fixed it yesterday.
None of these medications work on Alzheimer’s disease.
Paula, I’m not sure what you mean. Are you saying that none actually work on stopping the disease or are you saying that the drugs that have been around don’t work on the symptoms of the disease?